By using a super-computer to virtually squeeze and heat iron-bearing minerals under conditions that would have existed when the Earth crystallized from an ocean of magma to its solid form 4.5 billion years ago, two UC Davis geochemists have produced the first picture of how different isotopes of iron were initially distributed in the solid Earth.
The discovery could usher in a wave of investigations into the evolution of Earth’s mantle, a layer of material about 1,800 miles deep that extends from just beneath the planet’s thin crust to its metallic core.
"Now that we have some idea of how these isotopes of iron were originally distributed on Earth,” said study senior author James Rustad, a chancellor’s fellow and professor of geology, “we should be able to use the isotopes to trace the inner workings of Earth’s engine.”
A paper describing the study by Rustad and co-author Qing-zhu Yin, an associate professor of geology, was posted online by the journal Nature Geoscience on June 14 in advance of print publication in July.
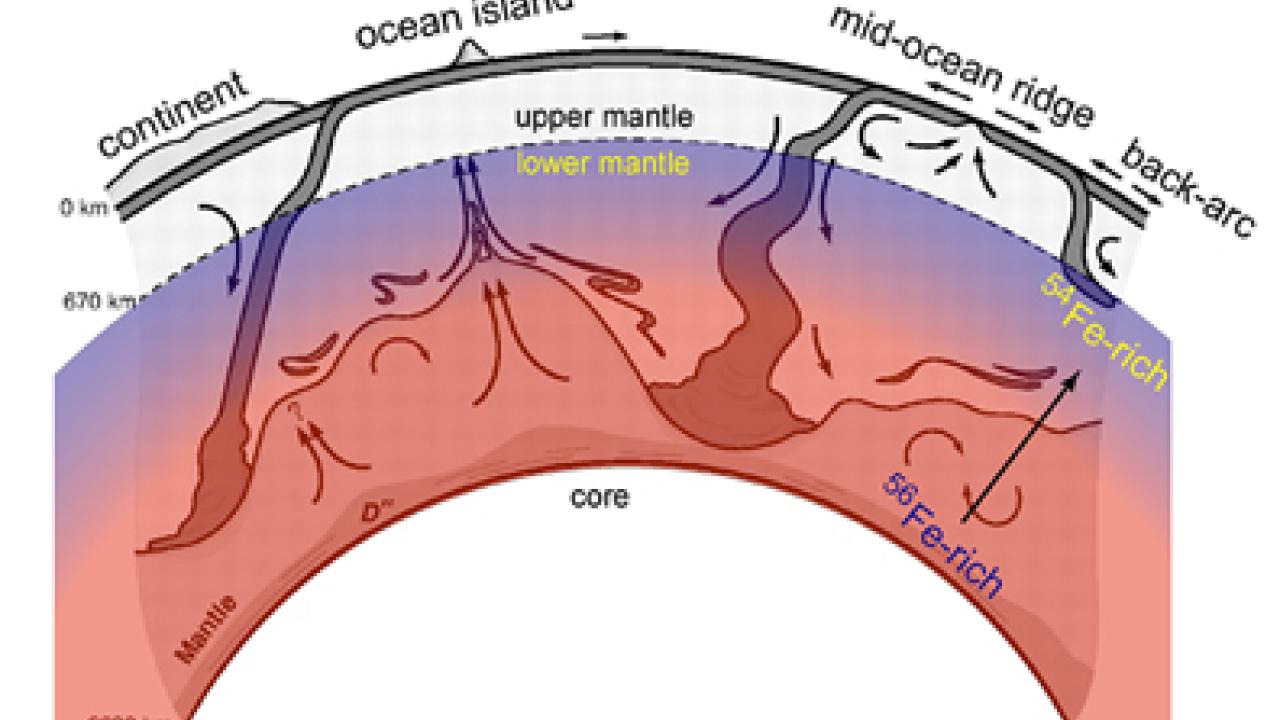
Sandwiched between Earth's crust and core, the vast mantle accounts for about 85 percent of the planet's volume. On a human time scale, this immense portion of our orb appears to be solid. But over millions of years, heat from the molten core and the mantle’s own radioactive decay cause it to slowly churn, like thick soup over a low flame. This circulation is the driving force behind the surface motion of tectonic plates, which builds mountains and causes earthquakes.
One source of information providing insight into the physics of this viscous mass are the four stable forms, or isotopes, of iron that can be found in rocks that have risen to Earth’s surface at mid-ocean ridges where seafloor spreading is occurring, and at hotspots like Hawaii’s volcanoes that poke up through the Earth’s crust. Geologists suspect that some of this material originates at the boundary between the mantle and the core some 1,800 miles beneath the surface.
“Geologists use isotopes to track physico-chemical processes in nature the way biologists use DNA to track the evolution of life,” Yin said.
Because the composition of iron isotopes in rocks will vary depending on the pressure and temperature conditions under which a rock was created, in principle, Yin said, geologists could use iron isotopes in rocks collected at hot spots around the world to track the mantle’s geologic history. But in order to do so, they would first need to know how the isotopes were originally distributed in Earth’s primordial magma ocean when it cooled down and hardened.
As a team, Yin and Rustad were the ideal partners to solve this riddle. Yin and his laboratory are leaders in the field of using advanced mass spectrometric analytical techniques to produce accurate measurements of the subtle variations in isotopic composition of minerals. Rustad is renowned for his expertise in using large computer clusters to run high-level quantum mechanical calculations to determine the properties of minerals.
The challenge the pair faced was to determine how the competing effects of extreme pressure and temperature deep in Earth’s interior would have affected the minerals in the lower mantle, the zone that stretches from about 400 miles beneath the planet’s crust to the core-mantle boundary. Temperatures up to 4,500 degrees Kelvin in the region reduce the isotopic differences between minerals to a miniscule level, while crushing pressures tend to alter the basic form of the iron atom itself, a phenomenon known as electronic spin transition.
Using Rustad’s powerful 144-processor computer, the two calculated the iron isotope composition of two minerals under a range of temperatures, pressures and different electronic spin states that are now known to occur in the lower mantle. The two minerals, ferroperovskite and ferropericlase, contain virtually all of the iron that occurs in this deep portion of the Earth.
These calculations were so complex that each series Rustad and Yin ran through the computer required a month to complete.
In the end, the calculations showed that extreme pressures would have concentrated iron’s heavier isotopes near the bottom of the crystallizing mantle.
It will be a eureka moment when these theoretical predictions are verified one day in geological samples that have been generated from the lower mantle, Yin said. But the logical next step for him and Rustad to take, he said, is to document the variation of iron isotopes in pure chemicals subjected to temperatures and pressures in the laboratory that are equivalent to those found at the core-mantle boundary. This can be achieved using lasers and a tool called a diamond anvil.
“Much more fun work lies ahead,” he said. “And that’s exciting.”
The work was supported by the U.S. Department of Energy's Office of Basic Energy Sciences, and by a NASA Cosmochemistry grant and NASA Origins of Solar Systems grants.
Media Resources
Dave Jones, Dateline, 530-752-6556, dljones@ucdavis.edu