A new technique sheds light on how red blood cells regulate blood pressure in small blood vessels.
A clear understanding of blood-pressure regulation could help development of treatments for diseases such as cystic fibrosis, pulmonary hypertension, diabetes and cancer. The researchers' findings appear online this month in the journal Proceedings of the National Academy of Sciences.
Human red blood cells are believed to play a key role in the regulation of blood pressure by means of an intricate mechanism that involves force from blood flow, biochemical signals and the protein configuration of the cell membrane. The details of this process, however, have been unclear.
"Not only will this new tool be important in developing a fundamental understanding of blood-cell behavior in response to different therapeutics, it also provides a way to probe how different foods may affect red blood cells' ability to micro-regulate blood pressure and, consequently, overall health," said co-author William Ristenpart, an assistant professor in the Department of Chemical Engineering and Materials Science.
Ristenpart, who has a joint appointment in the Department of Food Science and Technology, participated in this study as a postdoctoral fellow at Harvard University.
Human red blood cells can regulate blood pressure by releasing the chemical ATP (adenosine triphosphate), which triggers the blood vessel to expand. Previous studies have indicated that the release of ATP is related to "shear stress," the frictional force created by the flow of blood through the blood vessel. Although previous work suggested that shear stress deforms the shape of the red blood cells and triggers ATP release, it was not known just how much ATP was released by different levels of deformation.
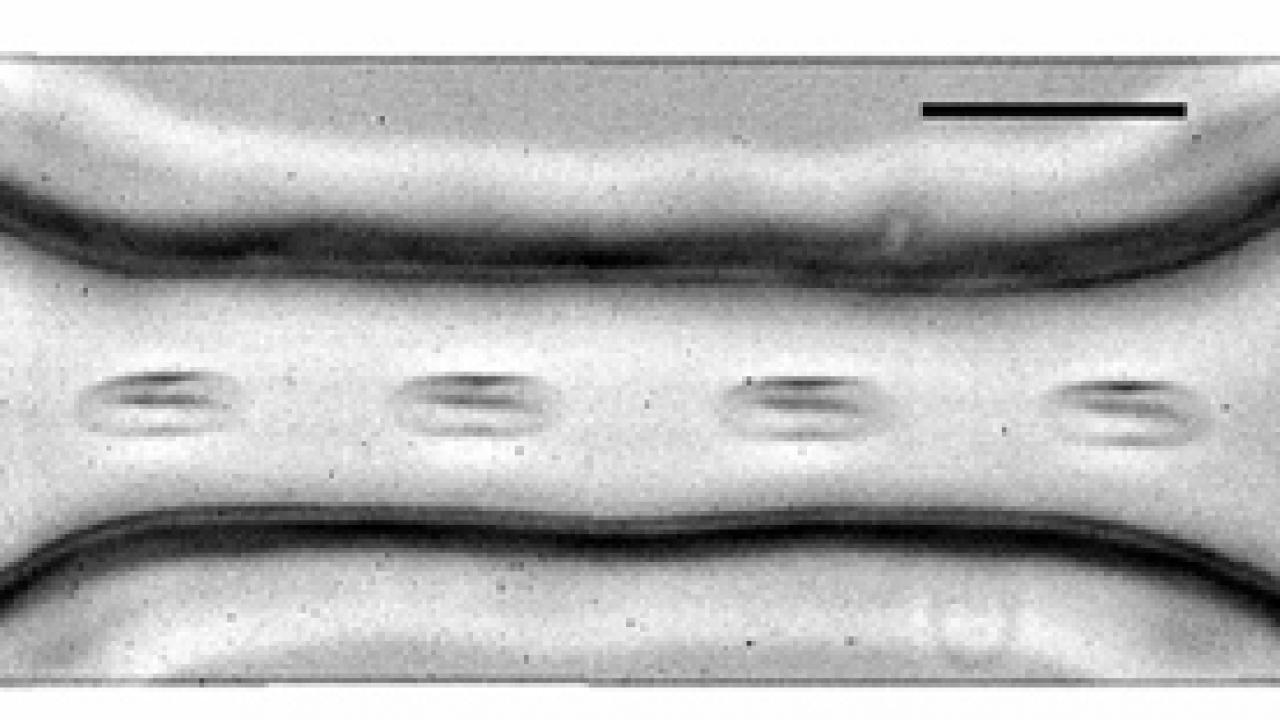
Ristenpart and colleagues Jiandi Wan and Howard Stone at Harvard University set out to study this process by forcing red blood cells through a microscopically small synthetic vessel that narrowed and then widened again, mimicking the passage of red blood cells through a constricted blood vessel. They used the light-emitting enzyme luciferase, which binds with ATP, to follow the timing and amount of ATP released by the red blood cells with millisecond resolution.
The researchers found that there was a considerable lag time from when the red blood cells first experienced increased shear stress to when they released ATP, and that an increase in shear stress resulted in a quicker and greater release of ATP.
The researchers also found that the length of time it takes the cell to change shape as it passes through a narrowed passageway signals how much ATP the cell should release. Contrary to their expectations, they found that elongation of the cells by as much as 40 percent was insufficient to trigger any ATP release if the elongation lasted less than 3 milliseconds.
The researchers suspect that the ATP-release mechanism is tied to the contraction and relaxation of the protein network that makes up the red blood cell's outer membrane.
Media Resources
Dave Jones, Dateline, 530-752-6556, dljones@ucdavis.edu